了解免疫缺陷状态下SARS-CoV-2进化和清除的变化
概要
在medRxiv预印本服务器上最近发布的一项研究中,研究人员对新冠患者进行了病毒免疫学分析,以描述持续感染的病毒学范围,并调查导致其发展的免疫学变量。
研究对象一共包括56名免疫抑制患者和184名免疫功能正常的新冠患者。免疫抑制组又分为非重症(NS)、重症自身免疫型/B淋巴细胞缺乏(SA)和重症血液肿瘤/移植(S-HT)亚组,其中包括31、13和12人。研究对这些患有不同类别免疫抑制疾病的个体上呼吸道的受试者的病毒动力学进行了检验。
研究显示,在具有SARS-CoV-2纵向序列的个体中,免疫抑制和非免疫抑制个体中分别有 39%和12%的SARS-CoV-2 S基因表现出核苷酸变化。严重免疫抑制的个体与SARS-CoV-2的更大进化以及抗SARS-CoV-2疗法耐药的风险增加有关。在免疫严重抑制的个体中,56% 的人出现mAb(单克隆抗体治疗)靶向耐药突变,其发生率明显高于其他群体。
研究强调了持续性COVID-19风险随免疫缺陷程度的变化,并表明由于体液和细胞介导的免疫抑制,SARS-CoV-2感染持续存在。
Understanding variations in SARS-CoV-2 evolution and clearance by immunodeficiency status
Immunosuppressed individuals have lower responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination and poorer coronavirus disease 2019 (COVID-19)-related consequences. Resistance to existing treatments, including targeted antibody therapy, is a concern posed by novel SARS-CoV-2 variants of concern (VOCs).
Immunosuppressed individuals exhibit persistent levels of SARS-CoV-2 for longer periods than non-immunosuppressed individuals, which might contribute to the emergence of new SARS-CoV-2 VOCs. To improve our understanding of the immunological risk factors and processes of SARS-CoV-2 persistence, large-scale investigations are needed.
In a recent study posted to the medRxiv preprint server, researchers perform viro-immunological analyses of COVID-19 patients to describe the virological range of persistent infection and investigate the immunological variables that contribute to its development.
Study: SARS-CoV-2 Viral Clearance and Evolution Varies by Extent of Immunodeficiency. Image Credit: HT-Pix / Shutterstock.com
About the study
The current study included 56 immunosuppressed and 184 immunocompetent COVID-19 patients who participated in the POSt-VaccInaTIon Viral CharactEristics Study (POSITIVES).
The immunosuppressed group was subdivided into the non-severe (NS), severe autoimmune-type/B lymphocyte-deficient (S-A) and severe hematologic-oncology/transplant (S-HT) subgroups, which comprised 31, 13, and 12 individuals, respectively. Viral dynamics in the upper respiratory tract among individuals with different categories of immunosuppressing conditions were determined.
The study participants provided six anterior nasal swab specimens over two weeks for SARS-CoV-2 ribonucleic acid (RNA) measurement using quantitative reverse transcription-polymerase chain reaction (PCR) and SARS-CoV-2 viral load assays. SARS-CoV-2 culture assays were also performed and SARS-CoV-2 neutralization was assessed.
SARS-CoV-2 spike (S) gene-specific next-generation sequencing (NGS) was used to quantify the number of unique intra-host single-nucleotide variants (iSNVs) in the S gene present at more than 3% frequency within every sample.
The dynamics of mutation emergence in the presence of monoclonal antibody (mAb) treatment were assessed. Among S-HT, S-A, NS, and non-immunosuppressed individuals, 10, nine, five, and 10 individuals, respectively, received mAb therapy.
Serum samples were obtained from the participants to characterize their humoral responses. Enzyme-linked immunosorbent spot (ELISpot) assays and antigen-targeted proliferation assays were used to elucidate T lymphocyte effector function. Generalized estimating equation (GEE) modelling was performed for the analysis.
Lack of viral clearance in severely immunocompromised patients contributes to SARS-CoV-2 mutations
Immunosuppressed individuals were significantly older as compared to non-immunosuppressed individuals with a median age of 55 and 46 years, respectively. Immunosuppressed patients were also more likely to receive antiviral and mAb therapy against SARS-CoV-2.
The study groups had a comparable median duration from the onset of symptoms or initial SARS-CoV-2-positive report to enrollment at five and four days, respectively.
Immunosuppressed and non-immunosuppressed individuals exhibited similar peak viral RNA levels. However, SARS-CoV-2 RNA clearance rates from nasal cavities varied significantly among the immunosuppressed groups, with S-HT individuals experiencing delayed SARS-CoV-2 elimination than other immunocompromised individuals.
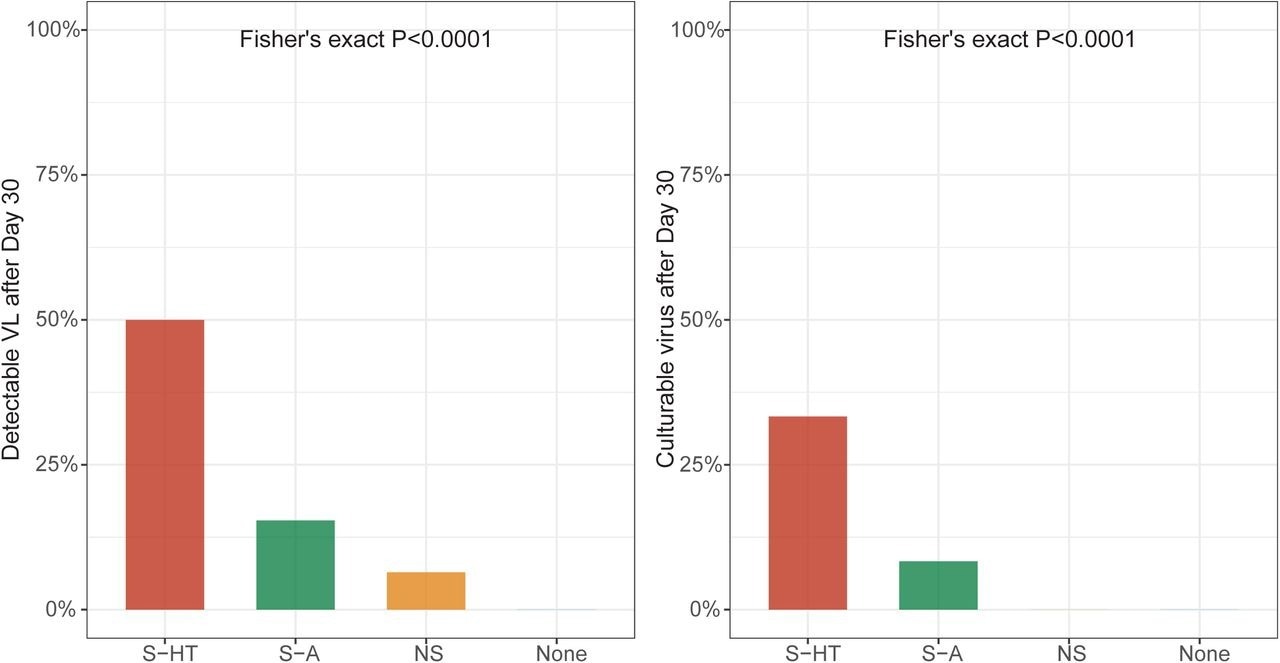
Detectable SARS-CoV-2 viral RNA (a) and culturable SARS-CoV-2 virus (b) beyond 30 days after symptom onset or first positive PCR/antigen tests, supplemental to Fig. 1. Fisher’s exact test was used to calculate the P values.
The median duration for nasal SARS-CoV-2 RNA clearance by S-HT individuals was 72 days, which was significantly longer than those by S-A, NS, and non-immunosuppressed individuals at seven, 11, and 11 days, respectively. Likewise, S-HT individuals exhibited significantly delayed culturable SARS-CoV-2 clearance at 21 days as compared to 6.5, six, and seven days for S-A, NS, and non-immunosuppressed individuals, respectively.
One month following the onset of COVID-19 symptoms or initial SARS-CoV-2-positive result, 6.5%, 15%, and 50% of NS, S-A, and S-HT individuals, respectively, showed SARS-CoV-2 presence as compared to none of the non-immunosuppressed individuals. Nucleotide and amino acid average pairwise distances were significantly greater in the S-A and S-HT groups than in the non-severe and immunocompetent groups.
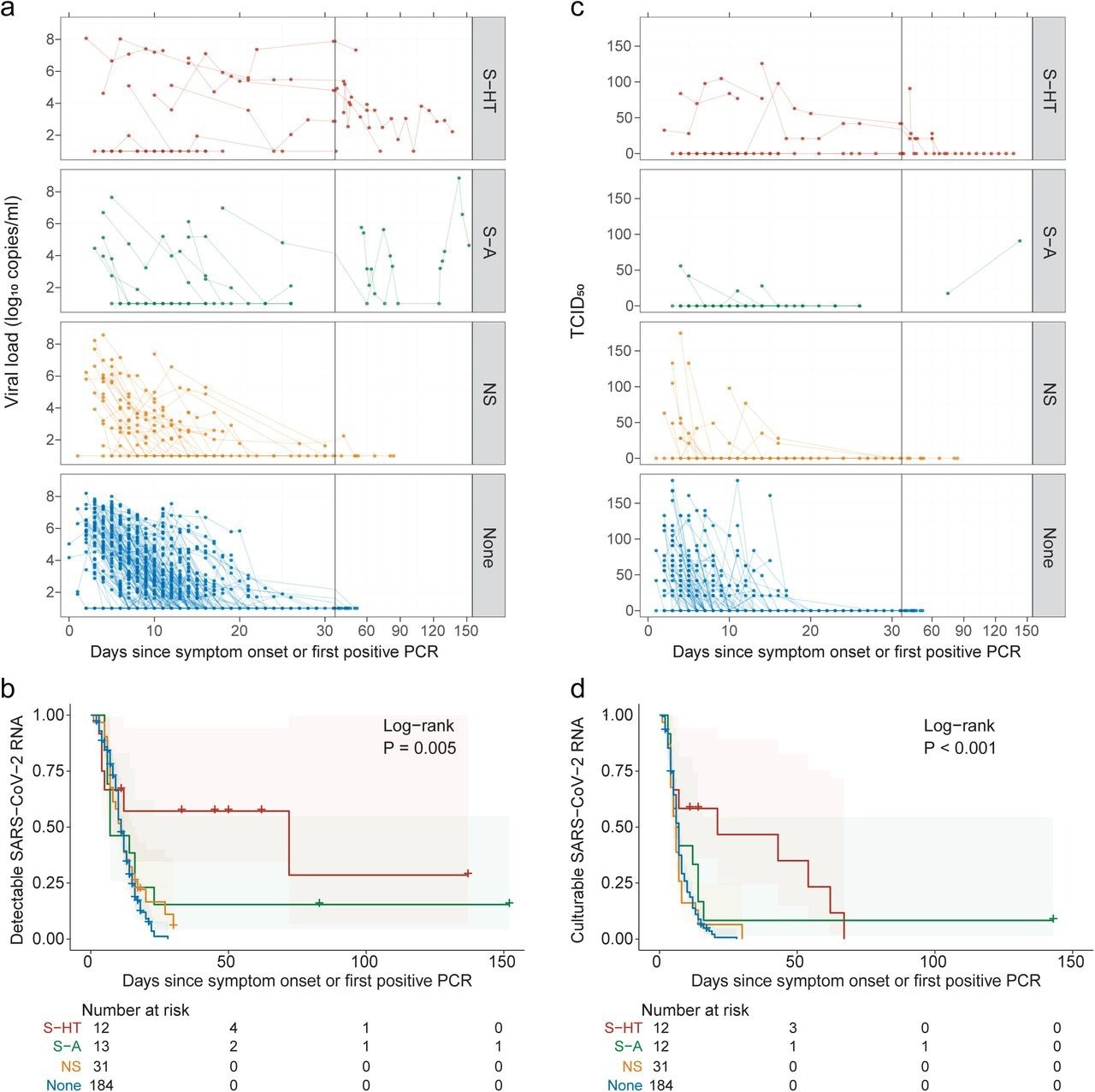
Kinetics of SARS-CoV-2 viral RNA and culturable virus among different immunocompromised groups a, Upper respiratory viral load decay. Lower level of quantification (LLOQ) is 10 copies/ml. b, Kaplan-Meier estimates of upper respiratory viral clearance (viral load below LLOQ). c, Upper respiratory culturable virus dynamics (50% Tissue Culture Infectious Dose [TCID50)). d, Kaplan-Meier estimates of upper respiratory culturable virus clearance.
Among individuals with longitudinal SARS-CoV-2 sequences, 39% and 12% of immunosuppressed and non-immunosuppressed individuals, respectively, exhibited nucleotide changes in the SARS-CoV-2 S gene. Severely immunosuppressed individuals were associated with a greater evolution of SARS-CoV-2 and increased risk of resistance to anti-SARS-CoV-2 therapies. Among severely immunosuppressed individuals, 56% developed mAb-targeted resistance mutations at a significantly higher rate than those observed in other groups.
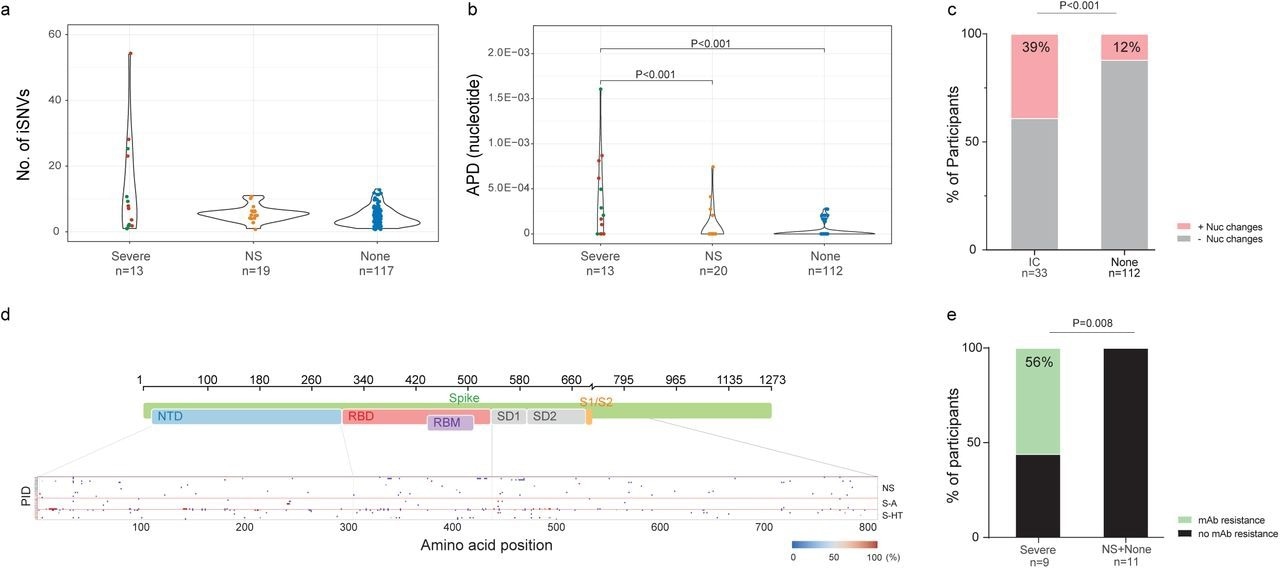
SARS-CoV-2 intra-host mutations among different immunocompromise groups.a, Number of intra-host single-nucleotide variants (iSNVs) among severe (S-HT in red and S-A in green), non-severe immunocompromised and non-immunocompromised (None) groups. b, Nucleotide average pairwise distance (APD) among severe (S-HT in red and S-A in green), non-severe immunocompromised (NS) and non-immunocompromised (None) groups. c, Participants with any nucleotide changes during follow-up. d, Heat map showing distribution of Spike polymorphisms from participants receiving mAb treatment. Each row represents one participant, while x axis shows amino acid positions in the Spike gene. Different domains of Spike are shown at the top. Colors indicate frequency of polymorphisms, with blue indicating the lowest value and red indicating the highest value in the scale. Participants in different study groups are separated by a red horizontal line. e, Proportion of mAb resistance emergence amongst those treated with mAbs, categorized by those with severe or non-severe/no immunosuppression. Comparison of iSNV and APD between groups were done using using Dunn’s test with Benjamini-Hochberg P value adjustment. Fisher’s exact test was used to calculate significance between participants with and without viral evolution and further, in participants with and without mAb treatment specific resistance mutations. Only significant P values are shown. NTD, N-terminal domain; RBD, receptor binding domain; RBM, receptor binding motif; S1, subunit 1; S2, subunit 2.
The S-A and S-HT individuals exhibited lowered SARS-CoV-2-specific antibody-mediated immunity, whereas only S-HT individuals showed decreased cell-mediated immunity.
At follow-up, non-immunosuppressed individuals exhibited significant increases in anti-Wuhan-Hu-1 and anti-variant SARS-CoV-2 S neutralizing antibody (nAb) levels, whereas nAb levels did not increase significantly among immunosuppressed individuals. Severely immunosuppressed individuals were found to mount 0.2-fold of the Wuhan-Hu-1 S-targeted antibody and 0.1-fold of the VOC S-targeted antibodies as compared to non-immunosuppressed individuals.
Similar findings were obtained for binding antibodies against the SARS-CoV-2 nucleocapsid protein. Non-immunosuppressed individuals exhibited lower interferon-gamma (IFN-γ) expression at zero to 14 days and 15 to 60 days following symptom onset or the initial SARS-CoV-2-positive test than the NS and S-A group individuals against SARS-CoV-2 Wuhan-Hu-1 strain and VOCs.
S-A individuals exhibited the highest cluster of differentiation (CD4+) and cytotoxic (CD8) T helper lymphocytic proliferation upon stimulation by SARS-CoV-2 S protein. S-HT individuals were associated with less functional T lymphocyte proliferation, despite IFN-γ secretions comparable with that by non-immunosuppressed individuals.
Conclusions
The study findings highlight variations in persistent COVID-19 risks by the extent of immunodeficiency and indicate that SARS-CoV-2 infection persists due to humoral and cell-mediated immune suppression.
Source:
News Medical
Published on 7 August, 2023
声明:本站文章版权归原作者及原出处所有。本文章系本站编辑转载,文章内容为原作者个人观点,登载该文章的目的是为了学习交流和研究,并不代表本站赞同其观点和对其真实性负责,本站只提供参考并不构成任何投资及应用建议。
本站是一个学习交流和研究的平台,网站上部分文章为引用或转载,并不用于任何商业目的。我们已经尽可能的对作者和来源进行了告知,但是能力有限或疏忽,造成漏登或其他问题,请及时联系我们,我们将根据著作权人的要求,立即更正或删除有关内容。本站拥有对本声明的最终解释权。








