The birth of anti-COVID-19 prevention drugs
Suntrap Life Technologies LTD. / Suntrap Health & Environment Research Institute
The COVID-19 has lasted for nearly two years, and the global incidence of COVID-19 continues to rise. As of December 7, 2021, the total number of confirmed cases worldwide has exceeded 266 million, and the total number of 5.27 million deaths has been reported. In the face of multiple rebounds in the global epidemic, rapid virus mutations, and rising rates of vaccine breakthrough infections, safe and effective drugs for COVID-19 treatment have become one of the breakthroughs in the epidemic. However, the research and development (R&D)of new drugs have the characteristics of a long cycle, large capital investment, and high failure rate. So far, safe and effective anti-COVID-19 drugs have not yet come out, and the development of anti-COVID-19 drugs is difficult and long.
Suntrap insists on disease-oriented research and the development of new drugs to serve patients and clinical needs.
To promote the R&D of new drugs and improve the ability of human beings to fight diseases. In recent years, Suntrap's research team has been cultivating new drug development systems and has gradually improved Suntrap's drug design and development systems. Suntrap's research team insists that disease-oriented drug development is the core of innovative drug R&D. Molecule-oriented drug R&D focuses on a certain molecule for a given drug target but ignores the patient's pathological characteristics, clinical needs, and even potential market prospects, which is prone to repeated research for both academia and industry and leads to a waste of resources. Meanwhile, disease-oriented drug R&D targets unmet clinical needs and provides potential clinical treatment schemes for certain diseases.
Suntrap's research team upholds the core appeal of drug development to serve patients and clinical needs, which is not only reflected in the social demands and research that fully consider the patient's pathological characteristics, clinical needs, pathogenic mechanism, but also involves in the processes of the determination of the R&D direction to the acquisition of related targets, molecular design, and screening, clinical trial development, etc. All processes of new drug R&D are comprehensively oriented by clinical and patient needs. Only in this way can rapid transform medical research into industrialization to realize the fundamental value of new drug R&D, that is, to address clinical needs and to maximize the benefits of society and patients. This is the basic core of the new drug R&D of the Suntrap research team.
Suntrap research team develops new anti-COVID-19 drug design.
In view of the indisputable fact that COVID-19 is ravaging around the world, Suntrap's research team immediately responded to the epidemic and then launched an anti-COVID-19 drug R&D project in January 2020. Suntrap's research team conducted in-depth and systematic investigations into the pathogenic mechanism of COVID-19. As we know, COVID-19 is composed of structural proteins and non-structural proteins, and the positive single-stranded RNA genome is wrapped by a structural protein envelope. Single-stranded RNA is unstable and RNA-dependent RNA polymerase (RdRp) does not have a nuclease proofreading activity. Therefore, its genome has a relatively high rate of nucleotide mismatches during the replication process and is prone to mutation. Based on these characteristics of COVID-19 and the commonality of coronavirus, the Suntrap research team formulated the R&D standards for the design and development of broad-spectrum anti-coronavirus drugs with multi-target synergy.
Meanwhile, Suntrap's research team accurately analyzed the pathological characteristics of COVID-19 clinical patients. In the face of moderate and severe patients with clinical illnesses mainly in the lungs, oral treatment drugs are difficult to directly reach the lungs, resulting in weakened or even ineffective drug effects. To this end, Suntrap's research team designed and developed aerosolized inhalation formulations of broad-spectrum anti-coronavirus drugs. The drug directly acts on the upper respiratory tract at the initial stage of infection to inhibit the activity of COVID-19 through aerosol inhalation. When the drug reaches the lungs, it exerts efficacy, synergistically regulates and repairs damaged cells, eliminates lung inflammation, and relieves and reverses pulmonary fibrosis. In response to the needs for epidemic prevention and control, based on aerosol inhalation preparations, the Suntrap research team urgently developed broad-spectrum anti-coronavirus prevention oral and nasal spray that is mainly for patients with mild or asymptomatic infection at the initial stage of COVID-19 infection. COVID-19 easily attaches to the mucosa of the mouth and nose to cause symptoms and is connected to the four cavities of the frontal, sphenoid, ethmoid, and maxillary sinuses through the nasal sinus opening. Besides, COVID-19 will hide in these four cavities and then replicate and spread in large numbers. Therefore, the purpose of broad-spectrum anti-coronavirus prevention oral and nasal sprays is to prevent the infection of COVID-19 and block its spread.
The difficulty of multi-targets drug design is that there are many limiting factors, and it is necessary to coordinate and balance various parameters to keep it in a moderate range. To design potential lead compounds that can be associated with multiple targets, Suntrap's research team combined or coupled several of the most critical targets in the pathogenesis of COVID-19, with the goal of making the total effects greater than the sum of the effects of each target. Suntrap's research team used the self-developed IDDNU® supercomputing and AI-aided drug design (AIDD) technology to balance the interactions between the targets of COVID-19, and then quickly to select potential compounds with good activity and low toxicity from the self-constructed drug resource libraries through a series of AI-based drug design processes, including the prediction of ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties, target- and COVID-19 infected cell-based drug inhibitions prediction models for virtual screening, and consistent scoring. These potential compounds were then subjected to cell-based phenotypic antiviral screening experiments. In May 2020, Suntrap's research team successfully discovered the lead compound "LeSoleil-T" that shows broad-spectrum anti-coronavirus effects with multi-targets.
Experiments prove that LeSoleil-T has inhibitory effects on COVID-19
To verify the inhibitory effects of LeSoleil-T on COVID-19, in August 2020, Suntrap commissioned the Guangdong Provincial Institute of Public Health (Guangdong Provincial Center for Disease Control, GPCDC) to conduct a study on the susceptibility of COVID-19 to "LeSoleil-T" (Figure 1).

Figure 1. Scanned image of the study report on the susceptibility of COVID-19 sensitivity study report onto LeSoleil-T issued by Guangdong Provincial Institute of Public Health
To test the cytotoxicity of LeSoleil-T, the experimenter administered different concentrations of LeSoleil-T to cells (African green monkey kidney cells Vero-E6). LeSoleil-T will not be toxic to cells within a certain concentration range. According to the experimental data, the TC50 value of LeSoleil-T is 121.06 μg/mL, indicating that LeSoleil-T has low toxicity.
In vitro, cell-based inhibition assays show that LeSoleil-T can effectively inhibit COVID-19 and prevent its damage to cells (Figure 2). Further analysis shows that LeSoleil-T has a high inhibition rate of 99.41% against COVID-19 at a concentration of 121.06 μg/mL. In addition, even if LeSoleil-T is at low concentrations (0.195-1.562 μg/mL), its inhibition rate against COVID-19 is greater than 63.06%, demonstrating that LeSoleil-T (LeSoleil) at low concentrations still has a high inhibitory on COVID-19.
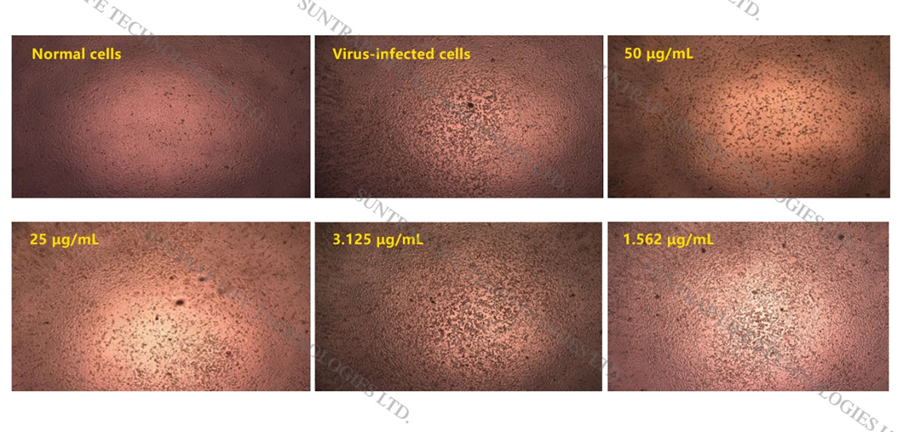 Figure 2. Photomicrographs of the susceptibility experiment of COVID-19 to LeSoleil-T (Source: Photomicrographs of the susceptibility experiment of COVID-19 to LeSoleil-T by the Guangdong Provincial Institute of Public Health.)
Figure 2. Photomicrographs of the susceptibility experiment of COVID-19 to LeSoleil-T (Source: Photomicrographs of the susceptibility experiment of COVID-19 to LeSoleil-T by the Guangdong Provincial Institute of Public Health.)
The official report issued by the Guangdong Provincial Institute of Public Health (Figure 3) shows that LeSoleil-T can effectively inhibit the replication of COVID-19 in the in vitro cell infection model, and the LeSoleil-T selection index (SI) value is greater than 1.0, with a foundation for further drug R&D against COVID-19.

Figure 3. Scanned picture of the conclusion of the COVID-19 sensitivity study report on LeSoleil-T issued by the Guangdong Provincial Institute of Public Health.
After clarifying that LeSoleil-T has the basis for drug development, the Suntrap research team subsequently demonstrated the relationship between toxicity, dosage, and biological efficacy, that is, exploring the limited dosage of LeSoleil-T in the formulation. LD50 is one of the important reference factors for the limited dosage of preparations. The experimental test results on the rat toxicity model show that the LD50 of LeSoleil-T is greater than 5 g/kg, indicating that LeSoleil-T is highly safe at the animal level. In November 2020, Suntrap's research team completed the formulation of broad-spectrum anti-coronavirus prevention and treatment oral and nasal spray formulations. Meanwhile, Suntrap's research team continues to conduct R&D verification of broad-spectrum anti-coronavirus aerosol inhalation preparations. It is foreseeable that broad-spectrum anti-coronavirus aerosol inhalation preparations for LeSoleil-T will become the first choice for the treatment of moderate and severe patients, due to its high safety and fast onset.
LeSoleil-T has attracted worldwide attention
Based on Suntrap's new drug design and development system and the accumulation of research by Suntrap's research team in the field of antiviral drug development over the years, Suntrap's research team can quickly identify and formulate lead compounds against COVID-19. It took less than six months for LeSoleil-T from conceptual design to lead compound discovery. The emergence of LeSoleil-T filled the gaps in the field of symptomatic COVID-19 and broad-spectrum anti-coronavirus drugs. The Suntrap research team will continue to explore innovative approaches for broad-spectrum anti-coronaviruses, providing more diversified possibilities for the prevention, control, and treatment of broad-spectrum coronaviruses.
LeSoleil-T has attracted widespread media attention from the beginning of its discovery. Since December 2020, domestic and foreign media such as China Net, US "Medical Daily", US "Bloomberg", French "Paris Daily", European "Postal Bulletin" and other 1425 media have successively reported on the breakthrough achievements made by Suntrap's research team in the development of new broad-spectrum anti-coronavirus drugs (Figure 4).

Figure 4. China Net (left picture), Bloomberg News (middle picture), and Medical Daily (right picture) reported on the research results of Suntrap's research team.
In January 2021, the application of LeSoleil-T in the preparation of anti-coronavirus drugs has been authorized a Chinese invention patent. Suntrap’s research team believes that drug development is meaningful only when it is applied to treat clinical diseases. Currently, Suntrap's research team is actively seeking clinical revalidation cooperation to further verify its clinical efficacy.
Up to now, the Delta strain has received global attention because of its greatly enhanced transmission and pathogenicity and its ability to severely reduce the immune effects of vaccines. According to data from Scripps College in the United States, there are about two new mutations in COVID-19 every month, and the current Delta strain has grown to 56 variant subtypes. Among them, the subtype variant AY.4.2 of the Delta strain has attracted great attention, because its infectivity is even 10% to 15% higher than that of the Delta strain. The existing vaccines are not sufficient to combat and eliminate the variant virus. The epidemic knows no borders, and the truly effective scientific prevention and control methods in the face of the virus depend on the discovery of anti-COVID-19 specific drugs. Multi-targets LeSoleil-T has broad-spectrum inhibitory effects on the original and mutant COVID-19 strains a multi-targets. Collectively, LeSoleil-T, a new broad-spectrum anti-coronavirus drug developed by the Suntrap research team can both prevent and treat COVID-19 and mutant strains. It is an inevitable way out under the situation of normalization of epidemic prevention and control and influenza of the COVID-19.
Suntrap's research team will continue to conduct in-depth research on the mechanisms of action for this drug. Based on the multi-targets effects of LeSoleil-T, we hope to develop more expanded drug formulations to treat potential sequelae caused by COVID-19 infection. Suntrap fully assumes the social responsibilities of a private scientific research enterprise, integrates tradition and innovation, considers the overall situation, overcomes numerous obstacles, does not use speculation as to the purpose, and contributes to the prevention and control of the COVID-19 epidemic and the protection of human health in a down-to-earth manner, and prepares for the possible emergence of virus mutation strains in the future. In the next report, the author will elaborate from the perspective of the mechanisms of action of LeSoleil-T, focusing on the multi-targets mechanisms and applications of LeSoleil-T.
Attachment 1: Timeline for the release of the research results on "Broad-spectrum anti-coronavirus drugs" of Suntrap's research team
January 29, 2020 "Emergency Launch of the ‘Research on Anti-Covid-19 Products’ Project"
May 17, 2020 "Suntrap S-CPDD New Drug Design and R&D System"
November 29, 2020 "Suntrap Life Technologies discovered a natural anti-COVID-19 compound"
February 7, 2021 "Through AI, Suntrap discovered ‘LeSoleil’, against COVID-19 and epidemic"
Attachment 2: Some media reports.
December 2020
Bloomberg.com "Suntrap Life Discovers Natural anti-COVID-19 Compound"
Dailyherald.com "Suntrap Life Technologies discovered a natural anti-COVID-19 compound"
Parisdaily.com « LeSoleil-T Suntrap Life Technologies a découvert un composé anti-COVID-19 naturel »
Post-gazette.com "Suntrap Life Technologies discovered a natural anti-COVID-19 compound"
MSN.hk.cn《針對新冠病毒,盛普始終堅持多靶點協同的防治病毒藥物研發》
February 2021
Bloomberg.com "Suntrap Discovered 'LeSoleil' for COVID-19 and the Epidemic"
One News Page.com "Suntrap Discovered 'LeSoleil' for COVID-19 and the Epidemic"
Sina.com《全球疫情仍旧严峻,盛普新冠肺炎治疗药研发有重大突破!》
hkrxw.com《全球疫情仍舊嚴峻,盛普新冠肺炎治療特效藥研發有重大突破!》
March 2021
April 2021








